- Home
- Case Studies
- Pharma Industry
Pharma Industry
Effluent Treatment Plant for Pharmaceutical Manufacturing
Pharmaceutical manufacturing produces some of the most complex and heavily regulated wastewater streams. With high COD, BOD, APIs (Active Pharmaceutical Ingredients), and toxic compounds, pharma effluent must be treated with precision.
At Geist, we offer specialized effluent treatment plants (ETPs) and Zero Liquid Discharge (ZLD) systems for the pharmaceutical industry, ensuring you meet environmental norms while recovering resources.
Why is Pharma Wastewater So Challenging?
Extremely high COD & BOD loads
Toxic, non-biodegradable substances
Presence of APIs and solvents
Tight discharge regulations (SPCB & CPCB)
Without proper treatment, pharma effluents can be harmful to both human health and the environment.
Geist’s ETP & ZLD Systems for Pharma Manufacturers
Geist provides end-to-end ETP and ZLD systems customized for pharma manufacturing units. Our designs handle:
High-strength effluent with fluctuating chemistry
Multiple waste streams from synthesis, formulation & washing
Strict norms for organic load, TDS, and residual APIs
We combine physical, chemical, and biological processes with advanced membrane technologies to meet ZLD goals.
Zero Liquid Discharge for the Pharma Sector
With increasing environmental regulations and water scarcity, ZLD in pharma is a smart choice. Geist’s ZLD solutions include:
Pre-treatment with pH correction, coagulation
RO + MEE + crystallizers for maximum water recovery
API filtration and advanced oxidation systems
Sludge management and condensate polishing
We help you recover up to 95% water and reduce environmental risk.
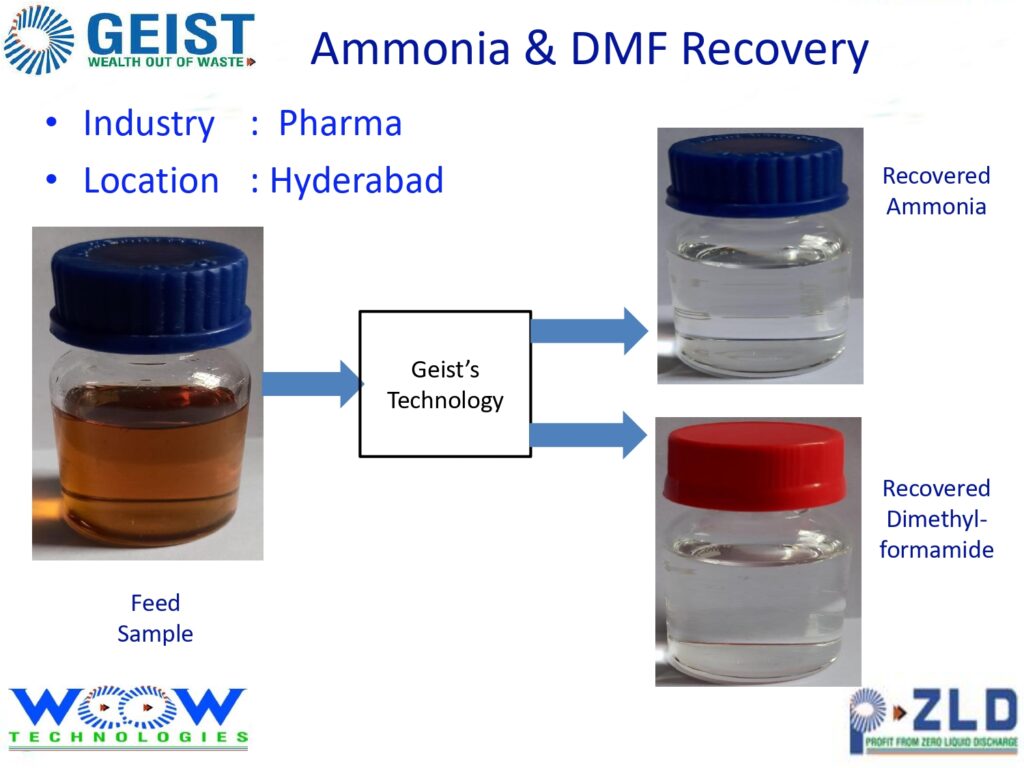
Resource Recovery & Cost Optimization
Geist also offers chemical recovery systems to help reclaim solvents and reduce treatment load. This adds value by:
Lowering fresh chemical consumption
Reducing sludge generation
Increasing operational ROI
Please feel free to reach out if you have any questions
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.